Dyne created the FORCETM platform to develop therapeutics that address the root cause of disease and deliver functional improvement for people living with neuromuscular disorders. The FORCE platform consists of an antigen-binding fragment (Fab), a linker and a payload that is rationally designed to target the genetic basis of the disease we seek to treat. We have a broad pipeline with clinical programs for myotonic dystrophy type 1 (DM1) and Duchenne muscular dystrophy (DMD), and preclinical programs for facioscapulohumeral muscular dystrophy (FSHD) and Pompe disease.
DYNE-101

PAYLOAD
Gapmer ASO for DMPK knockdown: DM1
DYNE-251
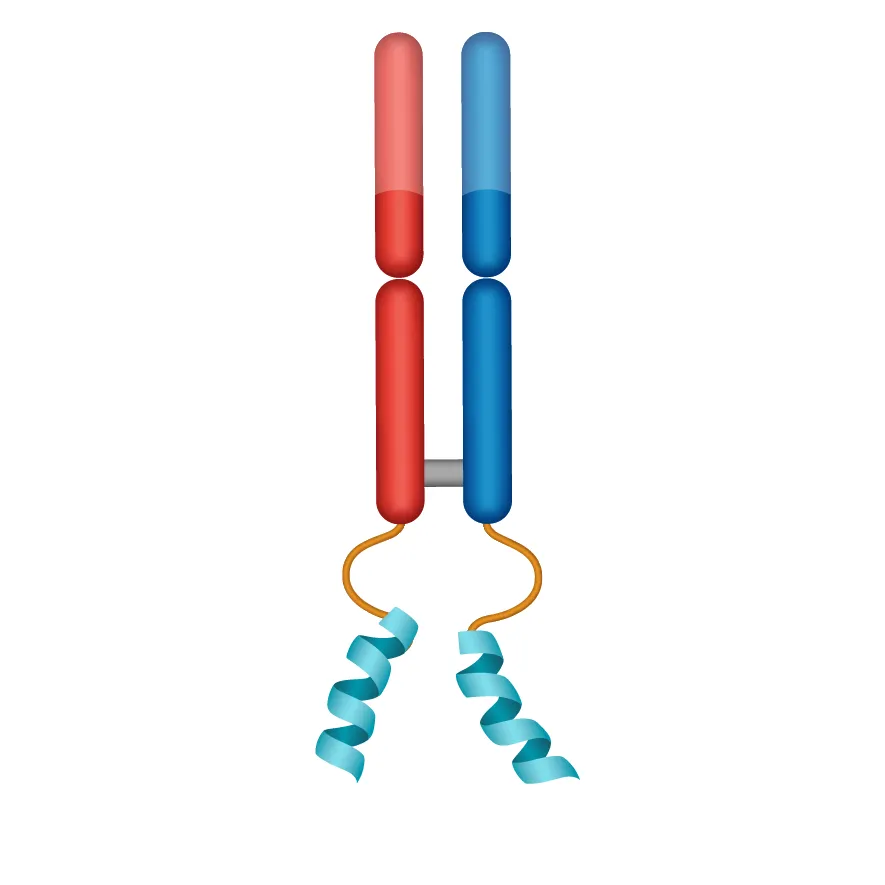
PAYLOAD
PMO for exon 51 skipping: DMD
DYNE-302
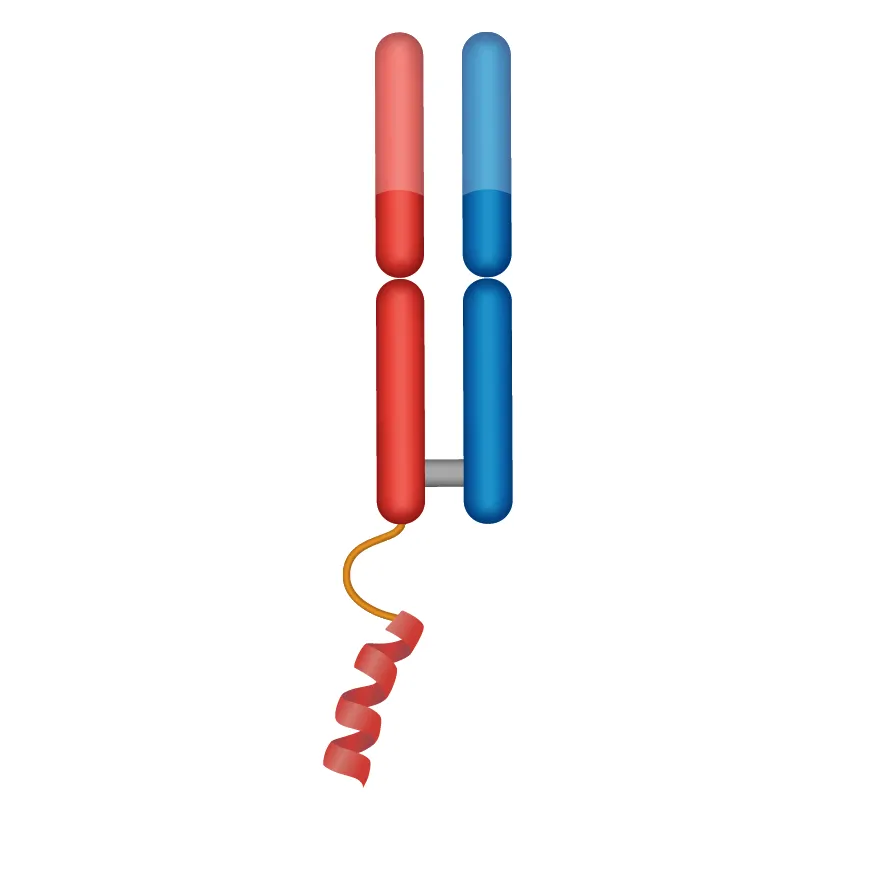
PAYLOAD
siRNA for DUX4 knockdown: FSHD
DYNE-401
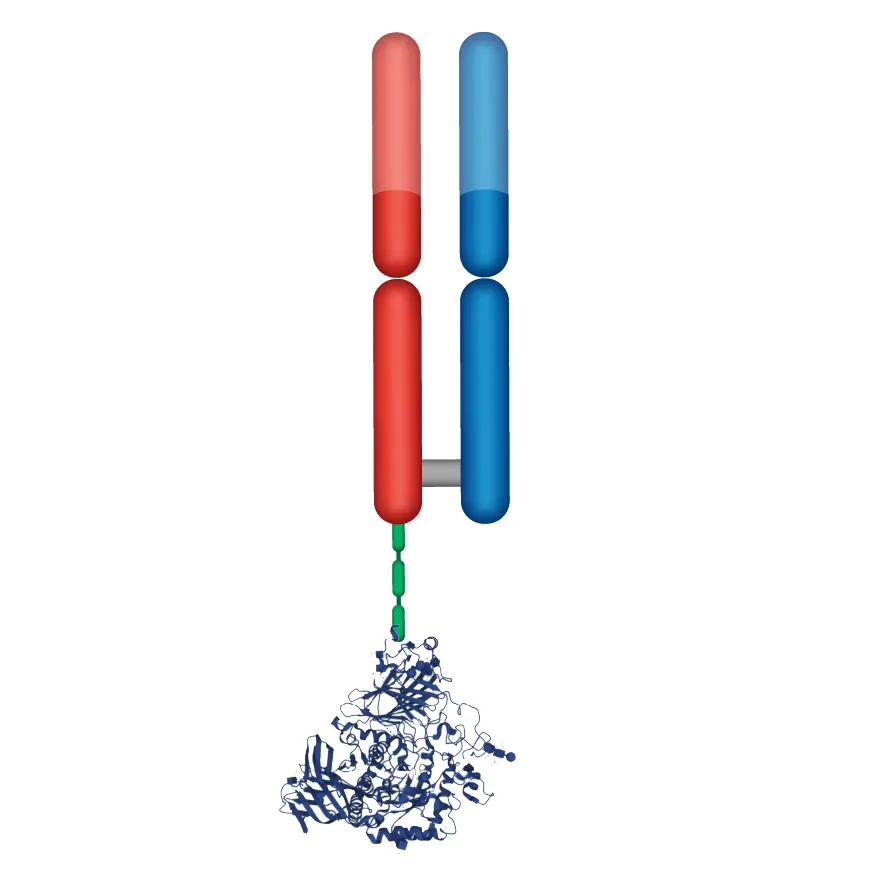
PAYLOAD
GAA enzyme replacement therapy (ERT): Pompe disease
The FORCE™ Platform
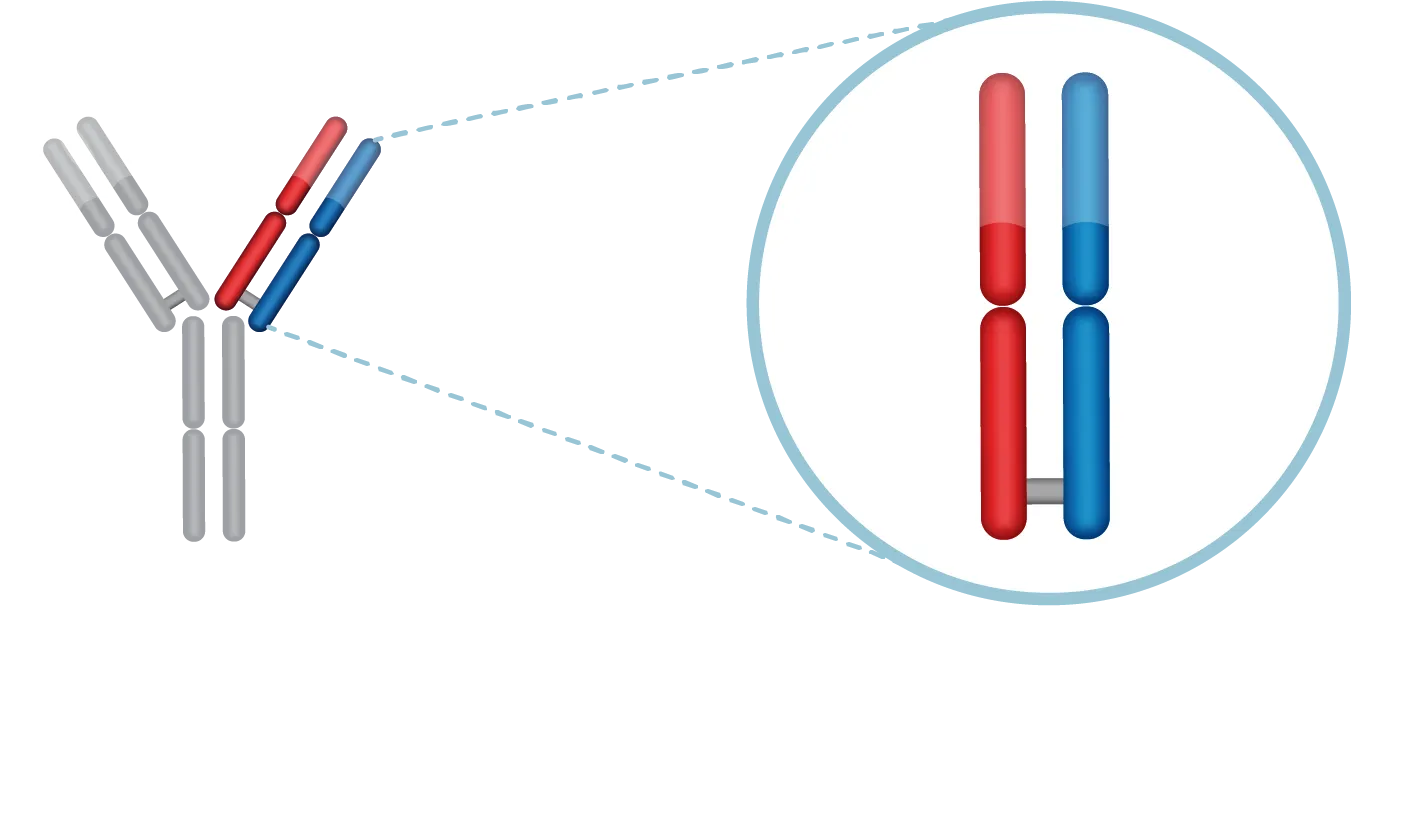
Our proprietary Fab binds to transferrin receptor 1 (TfR1) to enable delivery to muscle and the central nervous system (CNS) with the goal of providing functional improvement for people living with neuromuscular diseases. We believe a Fab offers significant advantages over a full antibody, including enhanced tissue penetration, increased tolerability and reduced risk of immune system activation.
Our Fab is linked to a therapeutic payload, such as an oligonucleotide or biologic, and the linker is chosen based on payload chemistry. The payload is carefully selected to directly address the root cause of the disease with the goal of delivering functional improvement.
We believe our FORCE platform provides several potential advantages, including delivery to muscle and CNS, extended time between doses and ability to re-dose.




